The Beckmann rearrangement is a fundamental organic chemical transformation that holds a significant place in the realm of organic chemistry. Named after the German chemist Ernst Otto Beckmann, this rearrangement is characterized by its capacity to convert ketoximes (compounds containing the =N-OH functional group) into amides or lactams—a process that involves the shifting of functional groups within the molecule.
The Beckmann rearrangement’s importance in organic chemistry cannot be overstated. It represents a key strategy for the conversion of functional groups in complex molecules, enabling chemists to tailor compounds to specific needs. This rearrangement serves as a versatile tool for the construction of a wide range of chemical structures, with applications spanning from drug synthesis to polymer chemistry.
Furthermore, the Beckmann rearrangement serves as an exemplar of the broader principles of reactivity, selectivity, and control that underpin organic synthesis. Understanding its mechanisms and applications provides valuable insights into the intricate world of chemical transformations and their profound impact on various industries and scientific disciplines.
The General Mechanism of the Beckmann Rearrangement
- Protonation of the oxygen atom in the oxime group by an acid catalyst, forming a resonance-stabilized intermediate.
- Migration of a neighboring alkyl or aryl group to the nitrogen atom, leading to the formation of an isocyanate intermediate.
- Nucleophilic attack of water or other nucleophiles on the isocyanate, resulting in the formation of an amide.
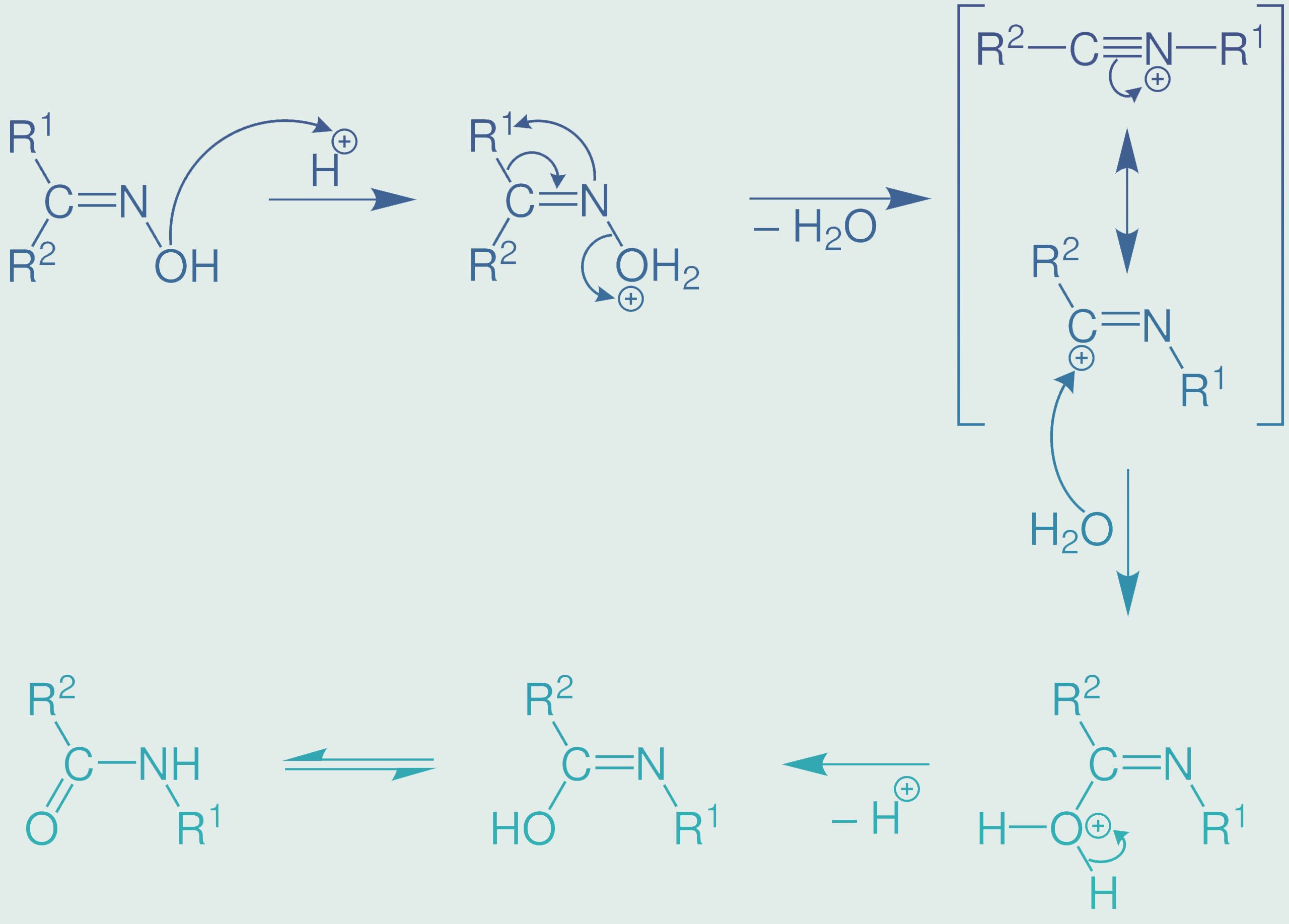
Mechanism of the Beckmann Rearrangement
Step-by-Step Reaction Mechanism
The mechanism of the Beckmann rearrangement involves a series of chemical steps that lead to the rearrangement of a ketoxime into an amide or lactam. Understanding this mechanism is essential for grasping the intricacies of this chemical transformation.
- Initiation: The process typically begins with the addition of an acid catalyst, such as sulfuric acid (H2SO4), to the ketoxime. The acid protonates the oxygen atom in the =N-OH group, forming a reactive oxonium ion.
- Rearrangement: The protonated oxygen subsequently attacks the adjacent carbon atom, leading to the formation of a cyclic intermediate called an isocyanate. This intermediate represents a critical juncture in the rearrangement process.
- Rearrangement Continues: The isocyanate intermediate undergoes further rearrangement, with the migration of an alkyl or aryl group. This migration is facilitated by the presence of the acid catalyst, and it results in the formation of an amide or lactam, depending on the starting ketoxime.
- Final Product Formation: The final step involves the deprotonation of the newly formed amide or lactam, yielding the desired product. The reaction often requires careful control of temperature and reaction conditions to ensure optimal yields.
Role of Acid Catalysis
Acid catalysis plays a pivotal role in the Beckmann rearrangement, serving as a catalyst that enhances the reaction rate without being consumed in the process. The acid catalyst serves several essential functions in this transformation.
- Protonation: The acid catalyst initiates the reaction by protonating the hydroxyl group (=N-OH) of the ketoxime. This protonation facilitates the formation of the reactive oxonium ion and sets the rearrangement process in motion.
- Intermediate Stability: Acid catalysis stabilizes key intermediates, such as the isocyanate, by facilitating protonation and deprotonation steps. This stabilization ensures that the rearrangement proceeds smoothly.
- Enhanced Reactivity: The presence of the acid catalyst increases the electrophilicity of the carbon atom involved in the rearrangement, making it more susceptible to nucleophilic attack and promoting the migration of functional groups.
- Reaction Control: Acid catalysis allows for precise control over reaction conditions, including temperature and reaction time, to optimize the formation of the desired amide or lactam product.
Factors Influencing Reaction Rate
Several factors influence the rate and efficiency of the Beckmann rearrangement. These factors are crucial considerations when planning and conducting this transformation.
- Nature of the Acid Catalyst: The choice of acid catalyst can significantly impact the reaction rate and selectivity. Different acids may exhibit varying reactivity and stability under the reaction conditions.
- Substrate Structure: The structure of the ketoxime substrate, including the nature of the substituents, can influence the ease and speed of the rearrangement. Bulky or sterically hindered groups may slow down the reaction.
- Temperature Control: Temperature plays a critical role in the Beckmann rearrangement. Fine-tuning the reaction temperature can lead to improved yields and selectivity.
- Reaction Time: The duration of the reaction is another parameter that affects the outcome. Longer reaction times may be necessary to achieve complete conversion, especially for complex substrates.
- Product Isolation: Considerations for product isolation and purification are essential, as side reactions or unwanted byproducts can occur. Proper techniques for product recovery are integral to the success of the rearrangement.
The primary starting material for the Beckmann Rearrangement is a ketoxime, which is an oxime derived from a ketone. This ketoxime is typically treated with an acid catalyst to undergo the rearrangement.
Applications and Uses of the Beckmann Rearrangement
The Beckmann rearrangement, with its ability to transform ketoximes into amides or lactams, finds diverse applications across various industries and scientific disciplines. Its utility extends far beyond the confines of a laboratory, making it a valuable tool for both research and practical applications.
Pharmaceutical Industry
- Drug Synthesis: The pharmaceutical sector heavily relies on the Beckmann rearrangement for the synthesis of vital compounds. It serves as a key step in the production of numerous pharmaceutical agents, including antibiotics, analgesics, and anti-cancer drugs. The rearrangement allows chemists to introduce essential amide or lactam functionalities into drug molecules.
- Functional Group Manipulation: Pharmaceutical researchers use the Beckmann rearrangement to strategically modify functional groups in drug candidates. This versatile transformation enables the creation of novel derivatives with enhanced biological activity and reduced side effects.
Synthesis of Amides and Lactams
- Versatile Amide Formation: The Beckmann rearrangement is a reliable method for the synthesis of amides, which are vital components in the chemical industry. Amides serve as building blocks for the production of plastics, polymers, and agrochemicals.
- Lactam Production: Lactams, cyclic amides with diverse applications, can be synthesized efficiently through this rearrangement. Lactams are utilized in the production of nylon, a versatile synthetic polymer, as well as in the synthesis of pharmaceuticals and herbicides.
Recent Advances and Innovations
- Green Chemistry Applications: The Beckmann rearrangement has seen adaptation in green chemistry practices, where the use of environmentally friendly reagents and conditions minimizes waste and energy consumption. Researchers continue to explore greener approaches to this reaction.
- Catalytic Developments: Ongoing advancements in catalysis have led to the development of more efficient and selective catalysts for the Beckmann rearrangement. These innovations enhance reaction yields and reduce the need for harsh reaction conditions.
- Applications Beyond Organic Chemistry: The versatility of the Beckmann rearrangement has led to its adoption in materials science, catalysis, and the development of functional materials. Its applications continue to expand into interdisciplinary fields.
Ernst Otto Beckmann was a German chemist known for his work in physical and organic chemistry. He made significant contributions to the understanding of reaction mechanisms and was instrumental in the development of the Beckmann Rearrangement, which bears his name. Beckmann’s research has had a lasting impact on the field of organic chemistry.
Experimental Techniques and Procedures
In the realm of experimental chemistry, the successful execution of the Beckmann rearrangement hinges on precise techniques and well-defined procedures. Ensuring optimal conditions and safety measures is paramount for reproducibility and desired outcomes.
Laboratory Setup
- Safety Measures: Before commencing the rearrangement, researchers must adhere to strict safety protocols. This includes the use of appropriate personal protective equipment (PPE) such as gloves, lab coats, and safety goggles. Adequate ventilation and fume hoods are essential when working with volatile or noxious reagents.
- Reagent Handling: Proper storage and handling of reagents is crucial. Researchers should ensure that all chemicals are of high purity and properly labeled. Special attention must be given to the handling of acid catalysts, which can be corrosive and hazardous.
- Equipment: A well-equipped laboratory is essential for conducting the Beckmann rearrangement. Standard laboratory glassware, including round-bottom flasks and condensers, is commonly employed. Temperature-controlled apparatus may be necessary to maintain the desired reaction conditions.
Common Reagents and Conditions
- Choice of Acid Catalyst: Sulfuric acid (H2SO4) is a widely used acid catalyst for the Beckmann rearrangement. Researchers may also explore alternative acids or catalytic systems, depending on the specific reaction requirements.
- Solvents: The selection of an appropriate solvent is crucial. Common solvents include dichloromethane (CH2Cl2), toluene (C7H8), and acetonitrile (CH3CN). The choice of solvent can impact reaction yields and selectivity.
- Reaction Temperature: Control of temperature is critical for the success of the rearrangement. Researchers typically carry out the reaction at elevated temperatures, often within the range of 120–160 °C, depending on the substrate and catalyst used.
Safety Considerations
- Acid Handling: Given the use of acid catalysts, researchers must exercise caution when handling reagents and performing the rearrangement. Accidental exposure to concentrated acids can lead to severe chemical burns.
- Ventilation: Adequate ventilation is essential to disperse any potentially harmful fumes generated during the reaction. Properly functioning fume hoods or a well-ventilated laboratory environment are imperative.
- Waste Disposal: The disposal of reaction byproducts and waste materials must follow established hazardous waste disposal guidelines. Proper containment and labeling of waste containers are essential.
- Emergency Response: Researchers should be well-versed in laboratory emergency response procedures, including the use of safety showers, eye wash stations, and fire extinguishers.
Various acid catalysts can be used in the Beckmann Rearrangement, including strong mineral acids such as sulfuric acid (H2SO4) and phosphoric acid (H3PO4). Acidic ion-exchange resins are also employed in some cases.
Notable Examples and Case Studies
Exploring real-world applications and case studies of the Beckmann rearrangement provides valuable insights into its practical significance and versatility across various industries.
Real-World Applications
- Pharmaceuticals: The pharmaceutical sector frequently employs the Beckmann rearrangement in drug synthesis. For example, it plays a pivotal role in the production of paracetamol (acetaminophen), a widely used pain reliever and fever reducer.
- Polymer Industry: The Beckmann rearrangement contributes to the production of nylon-6, a versatile synthetic polymer with applications in textiles, automotive components, and packaging materials.
- Agrochemicals: The synthesis of herbicides and insecticides often relies on the Beckmann rearrangement to introduce essential amide functionalities into the molecular structure.
Success Stories in Organic Synthesis
- Paracetamol Synthesis: The synthesis of paracetamol (acetaminophen) illustrates the practicality of the Beckmann rearrangement. By converting a ketoxime precursor into the desired amide, this transformation plays a crucial role in the mass production of this widely used pharmaceutical.
- Nylon-6 Production: The polymer industry extensively employs the Beckmann rearrangement to produce nylon-6, a durable and versatile material. This synthesis involves the conversion of a ketoxime into a lactam, which subsequently undergoes polymerization to form nylon-6.
- Crop Protection: In the agrochemical sector, the Beckmann rearrangement is instrumental in the synthesis of herbicides and insecticides. The introduction of amide functionalities enhances the efficacy and selectivity of these crop protection agents.
Challenges and Innovations
- Selective Rearrangement: Achieving high selectivity in the Beckmann rearrangement remains a challenge, especially when working with complex substrates. Researchers continually explore innovative catalytic systems and reaction conditions to enhance selectivity.
- Green Chemistry Advances: Ongoing efforts focus on developing greener and more sustainable variants of the Beckmann rearrangement. This includes the exploration of alternative solvents, catalysts, and energy-efficient methods.
- Complex Molecule Synthesis: The rearrangement’s adaptability in complex molecule synthesis is a subject of ongoing research. Chemists aim to leverage this transformation for the efficient construction of intricate structures in drug discovery and materials science.
Challenges and Future Directions
As the scientific community continues to explore the potential and limitations of the Beckmann rearrangement, several challenges have emerged, alongside promising avenues for future research and development.
Selectivity Enhancement
- Complex Substrates: Achieving high selectivity in the rearrangement, particularly when dealing with structurally complex substrates, remains a significant challenge. Researchers are actively seeking innovative catalytic systems and reaction conditions that can enhance selectivity, ensuring that the desired product is formed with precision.
- Chemo- and Regioselectivity: The Beckmann rearrangement often faces issues related to chemo- and regioselectivity. Overcoming these challenges is crucial for fine-tuning reactions and minimizing the formation of unwanted byproducts.
Green Chemistry Advancements
- Alternative Solvents: The development of greener and more sustainable variants of the Beckmann rearrangement involves the exploration of alternative solvents. Identifying environmentally friendly solvent options can reduce the environmental impact of the reaction.
- Catalytic Innovations: Researchers are actively investigating novel catalysts and catalytic systems that not only improve the efficiency of the rearrangement but also minimize the use of hazardous reagents. Catalytic advancements aim to align the reaction with green chemistry principles.
Complex Molecule Synthesis
- Drug Discovery: In the field of drug discovery and synthesis, the Beckmann rearrangement holds promise for efficiently constructing complex molecular structures. Researchers are working to harness this potential to streamline the synthesis of pharmaceutical compounds with intricate architectures.
- Materials Science: The versatility of the Beckmann rearrangement in materials science opens doors to the creation of advanced functional materials. Future directions include exploring its application in designing materials with tailored properties for various industrial applications.
Automation and High-Throughput Screening
- Robotic Synthesis: The integration of automation and robotics in chemical synthesis is a burgeoning area of research. Implementing automated systems for the Beckmann rearrangement can enhance efficiency, accuracy, and the rapid screening of reaction conditions.
- High-Throughput Screening: Researchers are exploring high-throughput screening techniques to expedite the discovery of optimal reaction conditions. This approach allows for the simultaneous testing of numerous catalysts and conditions, accelerating reaction optimization.
Interdisciplinary Collaboration
- Cross-Disciplinary Exploration: Collaboration between chemists, materials scientists, pharmacologists, and engineers is essential for unlocking the full potential of the Beckmann rearrangement. Interdisciplinary research can lead to innovative applications and solutions.
Education and Training
- Knowledge Dissemination: Disseminating knowledge about the Beckmann rearrangement and its applications is vital for future progress. Education and training programs can equip the next generation of researchers with the skills and insights needed to advance the field.
Sustainability and Safety
- Environmental Impact: Ongoing efforts to reduce the environmental impact of chemical processes, including the Beckmann rearrangement, are a focal point. Sustainable practices and the development of eco-friendly reagents contribute to the long-term viability of the reaction.
- Safety Protocols: Ensuring the safety of researchers and laboratory personnel remains a priority. Continued refinement of safety protocols and guidelines is essential to minimize risks associated with the rearrangement.
FAQs Beckmann Rearrangements
Can the Beckmann Rearrangement be used for the synthesis of polymeric materials?
What are some applications of the Beckmann Rearrangement in organic synthesis?
Are there any side reactions associated with the Beckmann Rearrangement?
Can the Beckmann Rearrangement be used for the synthesis of pharmaceuticals?
References
- Beckmann Rearrangements. An Investigation of Special Cases E. C. Horning, V. L. Stromberg, H. A. Lloyd J. Am. Chem. Soc., 1952, 74 (20), pp 5153–5155 doi:10.1021/ja01140a048
- Yamabe, S.; Tsuchida, N.; Yamazaki, S. (2005). “Is the Beckmann Rearrangement a Concerted or Stepwise Reaction? A Computational Study”. Journal of Organic Chemistry. 70 (26): 10638–10644. doi:10.1021/jo0508346.
- Li Tang, Zhi-Lv Wang, Hai-Lan Wan, Yan-Hong He, Zhi Guan. Visible-Light-Induced Beckmann Rearrangement by Organic Photoredox Catalysis. Organic Letters 2020, 22 (15) , 6182-6186. https://doi.org/10.1021/acs.orglett.0c02168.
- Ernst Otto Beckmann (1886). “Zur Kenntniss der Isonitrosoverbindungen” [On [our] knowledge of isonitroso compounds]. Berichte der Deutschen Chemischen Gesellschaft. 19: 988–993. doi:10.1002/cber.188601901222.