Curtius rearrangement, a fundamental chemical transformation in organic chemistry, stands as a remarkable and widely used reaction in this field. It represents a significant tool in the repertoire of synthetic chemists, enabling the conversion of carboxylic acid derivatives into primary amines, a crucial class of compounds with diverse applications.
The roots of the Curtius rearrangement can be traced back to its eponymous creator, Theodor Curtius, a German chemist who first described the reaction in the early 20th century. Over the years, this transformation has gained prominence due to its versatility and the myriad of compounds it can yield. Its enduring relevance in the realm of chemical synthesis is a testament to its efficacy and utility.
This article aims to provide a comprehensive understanding of Curtius rearrangement, from its underlying mechanisms and variations to its applications across various industries.
The General Mechanism of the Curtius Rearrangement
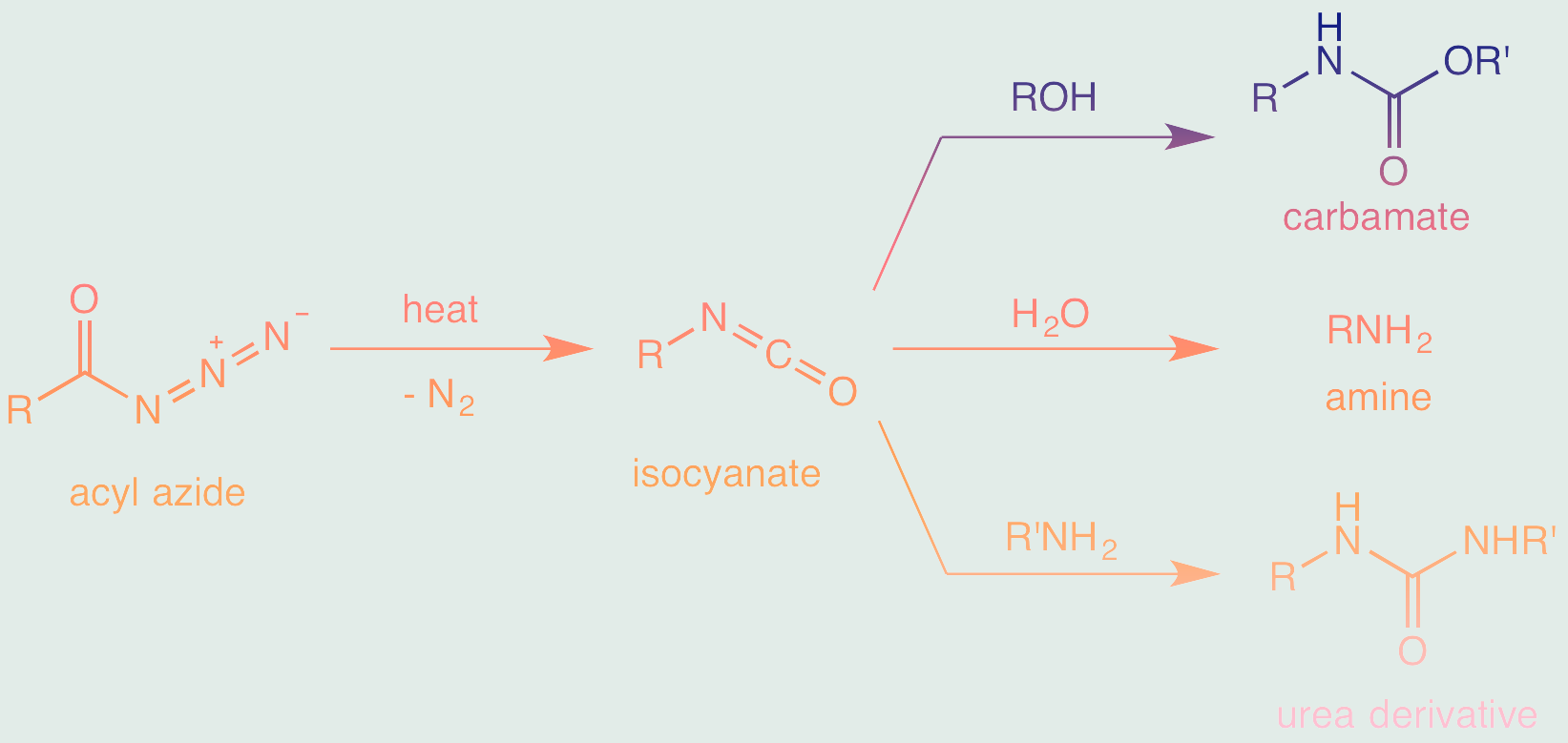
- Formation of the acyl azide from the corresponding acid chloride or carboxylic acid with azide ion (N3-) as a nucleophile.
- Rearrangement of the acyl azide to an isocyanate through migration of the R group.
- The isocyanate can further react with various nucleophiles to form a wide range of compounds.
Mechanism of Curtius Rearrangement
Formation of the Acyl Azide
The Curtius rearrangement begins with the conversion of a carboxylic acid derivative, typically an acyl chloride or an ester, into an acyl azide. This step involves the reaction of the starting material with a suitable azide source, such as sodium azide (NaN3). The nucleophilic attack of the azide ion on the carbonyl carbon of the starting compound leads to the formation of the acyl azide intermediate.
Rearrangement of the Acyl Azide
Upon the formation of the acyl azide, the reaction proceeds to the rearrangement stage. This rearrangement is initiated by thermal or catalytic activation, which induces the migration of a nitrogen atom from the azide group to the adjacent carbon atom, causing the formation of an isocyanate intermediate. The migration of nitrogen is a key feature of this reaction, leading to the transformation of the acyl azide into an isocyanate.
Isocyanate Rearrangement
The isocyanate intermediate undergoes a subsequent rearrangement, resulting in the formation of a carbamate intermediate. This rearrangement involves the migration of an oxygen atom from the carbonyl oxygen of the isocyanate group to the adjacent nitrogen atom. This step is pivotal in the conversion of the isocyanate into a carbamate, a key structural feature in the formation of primary amines.
Amine Formation
The final step of Curtius rearrangement involves the hydrolysis of the carbamate intermediate. Under suitable reaction conditions, typically in the presence of water or an aqueous solution, the carbamate is hydrolyzed, yielding the desired primary amine as the final product. This amine formation step is the culmination of the rearrangement process, resulting in the synthesis of a valuable compound from the original carboxylic acid derivative.
Understanding the stepwise mechanism of Curtius rearrangement is essential for chemists and researchers seeking to harness this powerful transformation in various synthetic applications. The conversion of carboxylic acid derivatives into primary amines through this mechanism has found widespread use in fields such as pharmaceuticals, materials science, and fine chemical production.
The main starting material for the Curtius Rearrangement is an acyl azide, which can be prepared from the corresponding acid chloride or other precursors. The reaction typically proceeds via the formation of an isocyanate intermediate.
Variations and Modifications
Curtius Rearrangement with Different Substrates
Curtius rearrangement is a versatile reaction that can be adapted to various substrates, expanding its applicability in synthetic chemistry. While the typical reaction involves carboxylic acid derivatives, variations have been developed to accommodate different starting materials. For instance, reactions with acyl chlorides, acid anhydrides, and esters have been well-documented. These variations allow chemists to tailor the reaction to specific synthetic needs, making Curtius rearrangement a valuable tool in organic synthesis.
Notable Reaction Conditions
The success of Curtius rearrangement often depends on reaction conditions. Researchers have explored a range of conditions to optimize the reaction for different substrates and desired products. Reaction parameters such as temperature, solvent choice, and the use of catalysts play crucial roles in achieving high yields and selectivity. Understanding these conditions is essential for harnessing the full potential of Curtius rearrangement in diverse applications.
Recent Developments and Innovations
Advancements in synthetic chemistry have led to innovative variations of Curtius rearrangement. Recent research efforts have focused on developing more efficient and sustainable reaction protocols. Green chemistry approaches, which emphasize environmentally friendly reagents and reduced waste generation, have gained prominence. Additionally, the integration of flow chemistry techniques has streamlined the process, enabling faster reactions and scalability. These developments represent the evolving landscape of Curtius rearrangement and its continued relevance in modern organic synthesis.
Theodor Curtius was a German chemist known for his pioneering work in organic chemistry. He made significant contributions to the understanding of diazo compounds and is best known for describing the Curtius Rearrangement, which is an important reaction in organic synthesis.
Applications in Organic Synthesis
Synthesis of Amines
Curtius rearrangement is a versatile tool in the synthesis of primary amines, a class of compounds with diverse applications. By converting carboxylic acid derivatives into amines, this reaction provides access to valuable intermediates for pharmaceuticals, agrochemicals, and specialty chemicals. The ability to control the structure and functionality of the resulting amines makes Curtius rearrangement a valuable asset in organic synthesis.
Pharmaceutical Industry
In the pharmaceutical industry, the synthesis of complex molecules often relies on efficient routes to primary amines. Curtius rearrangement finds extensive use in pharmaceutical synthesis, enabling the construction of amine-containing drug candidates. Its versatility allows researchers to access a wide range of amine structures, contributing to drug discovery and development.
Fine Chemicals Production
The production of fine chemicals, including fragrance compounds, dyes, and specialty polymers, benefits from Curtius rearrangement. This reaction pathway offers a streamlined approach to introducing amine functionality into molecules, facilitating the synthesis of high-value compounds. The ability to modify the reaction conditions and substrates allows for tailored solutions in fine chemical manufacturing.
Materials Science
Curtius rearrangement plays a role in materials science, particularly in the synthesis of polymers and advanced materials. By incorporating primary amines into polymer structures, researchers can engineer materials with specific properties, such as improved adhesion, biocompatibility, or electrical conductivity. The controlled introduction of amines via Curtius rearrangement contributes to the development of innovative materials with tailored characteristics.
Challenges and Limitations
Side Reactions and Byproducts
While Curtius rearrangement is a powerful synthetic tool, it is not without its challenges. One common issue is the potential for side reactions and the formation of unwanted byproducts. These side reactions can result in lower yields and complicate the purification process. Researchers must carefully optimize reaction conditions to minimize these undesirable outcomes and maximize the selectivity of the desired product.
Selectivity Issues
Achieving selectivity in Curtius rearrangement can be a complex task, particularly when dealing with substrates that contain multiple functional groups. Controlling which part of the molecule undergoes rearrangement can be challenging. Chemists often employ protecting groups and carefully designed reaction conditions to address selectivity issues. Nevertheless, achieving high selectivity remains a key consideration in the successful application of this reaction.
Safety Considerations
Curtius rearrangement typically involves the use of azides, which can be potentially hazardous compounds. Azides are known to be sensitive to heat and shock, and mishandling can lead to accidents. Researchers must exercise caution when working with azides, implementing safety protocols, and using appropriate protective equipment to mitigate risks. Safety awareness and proper training are essential aspects of conducting Curtius Rearrangement safely and effectively.
Future Prospects and Research Directions
Emerging Trends in Curtius Rearrangement
As the field of organic synthesis continues to evolve, Curtius rearrangement remains at the forefront of innovative chemical transformations. Emerging trends suggest a growing interest in expanding the scope of this reaction to accommodate new substrates and reaction conditions. Researchers are exploring novel reagents and catalysts to enhance reaction efficiency and selectivity, opening doors to previously challenging transformations.
Areas of Ongoing Research
Ongoing research efforts in Curtius Rearrangement are diverse and dynamic. Scientists are delving into the development of more sustainable and environmentally friendly reaction protocols. Green chemistry principles are guiding the exploration of eco-friendly alternatives to traditional reagents and solvents. Additionally, interdisciplinary collaborations are flourishing as researchers seek to apply Curtius rearrangement in fields beyond organic synthesis, such as materials science and biochemistry.
Potential Breakthroughs
The future of Curtius Rearrangement holds the promise of potential breakthroughs in several areas. Enhanced understanding of reaction mechanisms and intermediates may lead to more precise control over selectivity, enabling the synthesis of complex molecules with greater efficiency. Moreover, the integration of computational chemistry and artificial intelligence may accelerate reaction optimization and predictive modeling, streamlining the design of novel Curtius rearrangement processes.
Conclusion
In conclusion, Curtius rearrangement stands as a venerable and indispensable tool in the arsenal of synthetic chemistry. Its historical roots trace back to the early 20th century, and its relevance has only grown with time. This reaction, which facilitates the conversion of carboxylic acid derivatives into primary amines, has found applications across diverse industries, from pharmaceuticals to materials science.
Curtius Rearrangement, with its rich history and ongoing relevance, exemplifies the dynamic nature of organic synthesis. As researchers and chemists continue to explore its potential, this reaction remains poised to shape the future of chemical science and its applications in various industries.
FAQs Curtius Rearrangement
What are some applications of the Curtius rearrangement in organic synthesis?
Are there any side reactions associated with the Curtius rearrangement?
Can the Curtius rearrangement be used for the synthesis of pharmaceuticals?
Are there any safety considerations associated with working with azides, which are involved in the Curtius rearrangement?
Can the Curtius Rearrangement be used for the synthesis of polymers?
Are there variations or modifications of the Curtius Rearrangement?
References
- Rauk, A.; Alewood, P. F. (1977). “A theoretical study of the Curtius rearrangement. The electronic structures and interconversion of the CHNO species”. Can. J. Chem. 55 (9): 1498–1510. doi:10.1139/v77-209.
- L’Abbe, G. (1969). “Decomposition and addition reactions of organic azides”. Chem. Rev. 69 (3): 345–363. doi:10.1021/cr60259a004.
- Yukawa, Y.; Tsuno, Y. (1959). “The decomposition of substituted benzazides in acidic solvents, the acid catalysis”. J. Am. Chem. Soc. 81: 2007–2012. doi:10.1021/ja01517a055.